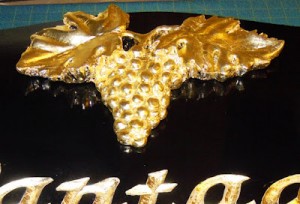
Exposed to the elements, many metals oxidize. When they do, they change colors. Copper and bronze will sometimes develop an attractive verdigris of green. As they weather, metals will also darken, turning their brassy colors to antique hues of brown. The term most frequently used for the weatherworn coloration that metals develop is patina.  With the application of a little chemistry, you can replicate the effect in a few simple steps without having to wait for Mother Nature to do her thing. There are many different recipes for creating a patina on a gilded surface. In fact, voluminous books have been published on the subject. The Society of Gilders journal, The Gilder’s Tip, will also occasionally have articles on chemical patination. Many of the chemical formulations are toxic or hazardous to your health. When working with chemicals, it is prudent to follow some basic safety precautions:
With the application of a little chemistry, you can replicate the effect in a few simple steps without having to wait for Mother Nature to do her thing. There are many different recipes for creating a patina on a gilded surface. In fact, voluminous books have been published on the subject. The Society of Gilders journal, The Gilder’s Tip, will also occasionally have articles on chemical patination. Many of the chemical formulations are toxic or hazardous to your health. When working with chemicals, it is prudent to follow some basic safety precautions:
- · Always read and heed the safety instructions pertaining to the product that you are using.
- · Many chemical formulation produce harmful or toxic gases. Always work in a well-ventilated area.
- · Always wear chemical gloves.
- · Keep as much of your skin covered.
- · Always wear safety glasses.
Liver of sulfur is available in a few different forms: dry chunks, premixed solution and a gel. The drawback of the dry chunks is that it has a shelf life. In solid form, liver of sulfur can rapidly degrade and lose its potency when it is exposed to oxygen.